Freeze drying, also called lyophilization, is an important process in the pharmaceutical world. It helps remove water from medicines to make them last longer and stay effective. This blog will walk you through the main stages of freeze drying and explain why careful control at every step is so important. If you’re curious about how delicate medicines are kept safe and stable, you’re in the right place!
What is Freeze Drying (Lyophilization)?
Freeze drying, or lyophilization, gently removes water from heat-sensitive products to help preserve them. Sublimation is the process where ice transitions directly into vapour without becoming liquid—a crucial step in maintaining the structural and chemical integrity of delicate substances.
This method is especially important in pharmaceuticals, where many biologics, injectables, and heat-sensitive drugs cannot tolerate conventional drying processes. By operating at low temperatures, freeze drying ensures the product remains stable, sterile, and effective for extended periods. Understanding Freeze Drying
Understanding the Freeze-Drying Process
The freeze-drying process is a multi-stage operation involving precise control over temperature and pressure. The core idea is to remove moisture while preserving the product’s original characteristics gently. The system comprises several key components
Product Chamber:
The main unit where the product is first frozen and then dried in a vacuum
Condenser:
A low-temperature surface that traps water vapour by converting it back into ice, preventing it from re-entering the product.
Vacuum System:
Reduces the pressure inside the chamber, enabling sublimation by lowering the boiling point of water.
Refrigeration System:
Keeps the chamber and condenser at low temperatures necessary for freezing and trapping water vapour.
Heating System:
Applies controlled heat to drive sublimation without damaging the product. These components work together in a series of stages that define the freeze-drying process.
The Stages of Freeze Drying
The freeze-drying process can be broken down into four main stages, each critical for ensuring the product’s quality and stability.
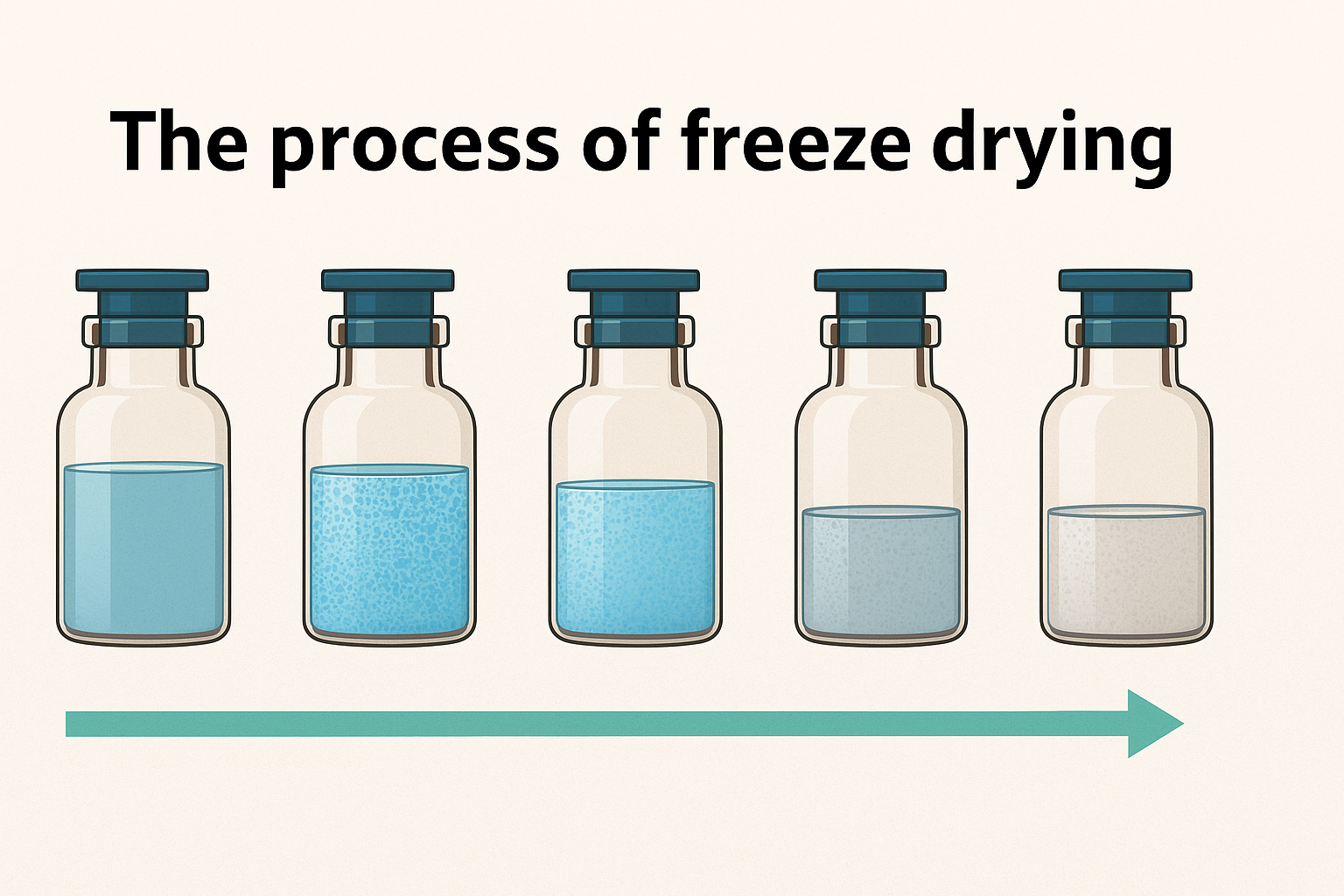
Stage 1: Freezing
The first step involves lowering the shelf temperature within the product chamber until the solvent, primarily water, freezes. This is crucial because the product itself has a lower freezing point. Thus, the temperature is decreased even further until the entire solution is frozen solid. During this phase, the product’s integrity is maintained by careful temperature control.
Stage 2: Primary Drying
Once the product is frozen, we transition to the primary drying phase. Here, the isolation valve opens, and a vacuum pump removes air from both the product chamber and the condenser. This reduction in pressure allows for the next critical process, sublimation. As the temperature of the shelves is gradually increased, the ice crystals in the product turn directly into vapor without passing through a liquid state.
This vapor then rises and exits the vial, leaving behind a partially dried product matrix. The water vapor travels through the isolation valve and is trapped on the cold condenser coils, effectively removing about 90% of the water content from the product.
Stage 3: Secondary Drying
Even after primary drying, the product is not completely dry. There remains some water bound to the product that requires additional heat to remove. In the secondary drying phase, the temperature is slowly increased further, allowing for the removal of this bound water. This step is crucial as it leaves behind a fully dried product matrix, ready for storage and use.
Stage 4: Sealing and Packaging
After the drying process is complete, sterile air is backfilled into both chambers to equalize the pressure. Following this, the freeze dryer collapses the shelves to insert stoppers fully. An over-seal is then placed over the stopper to ensure sterility before the product is packaged for transport. It’s essential to note that the product must be reconstituted before use in healthcare settings, ensuring that patients receive effective medications.
The Importance of Precision in Freeze Drying
Throughout the entire freeze-drying process, specific parameters such as temperature, pressure, and time are critical. These parameters are often tailored to the individual product’s formulation. The control of these factors is what ensures the integrity and effectiveness of the final product. Any deviation could lead to suboptimal results, potentially compromising the drug’s stability and efficacy.
Temperature Control
Temperature plays a vital role in both freezing and drying phases. Maintaining low temperatures during the initial freezing phase is essential to prevent the formation of large ice crystals, which can damage the product. During drying, careful temperature management allows for the effective sublimation of ice, ensuring that the product retains its desired properties.
Pressure Management
Similarly, managing pressure is crucial. The vacuum system must create a sufficiently low-pressure environment to facilitate sublimation. This pressure drop allows the ice to vaporize directly, bypassing the liquid state, which is essential for preserving the product’s chemical structure.
Applications of Freeze Drying in Pharmaceuticals
Freeze drying is widely used in the pharmaceutical industry for various applications. Here are some notable examples:
- Vaccines: Many vaccines require freeze-drying to ensure stability and longevity.
- Antibiotics: Freeze drying helps maintain the efficacy of antibiotics over time.
- Biologics: Protein-based drugs often undergo freeze-drying to preserve their structure and function.
- Diagnostic Kits: Many diagnostic reagents are freeze-dried for easy transport and storage.
Conclusion
Freeze drying, or lyophilization, is a complex but essential process in the pharmaceutical industry. From the initial freezing to the final sealing and packaging, every step must be meticulously controlled to ensure the stability and efficacy of the drug products. As the demand for stable and effective pharmaceuticals continues to grow, understanding and optimizing the freeze-drying process will remain a critical focus for the industry.
For those interested in learning more about freeze drying, consider exploring additional resources or online courses that cover the fundamentals and advanced aspects of lyophilization.
Relative Blogs: